
The expanding complexity of the phosphorylation and proteolytic signaling axis of MST1
The mammalian STE20-like kinases 1 and 2 (MST1 and MST2) have been widely investigated with their identified roles in the Hippo signaling pathway which regulates organ size (1) through the regulation of the TAZ and YAP effectors (2). While diverse cellular networks are impacted by the Hippo signaling pathway, including diverse signaling networks and global microRNA biogenesis (3,4), novel functions for the MST proteins emerge upon proteolysis of these kinases.
The activation of proteolytic pathways during apoptosis intersects many signaling pathways where a variety of kinases are cleaved (5), which leads to alternative roles for these kinases during cell death. An example of a kinases which exhibits distinct cellular functions upon proteolysis are MST1/2. Here we summarize the literature on the caspase activated forms of the MST kinases and highlight a recent report by Servas et al. (6) which reveals a novel signaling axis involving caspase activated MST1 and the anti-apoptotic casein kinase II (CK2).
The activation of the apoptotic program leading to the activation caspases has been demonstrated to lead to the cleavage of MST1. Two caspase cleavage sites have been mapped in MST1 (Figure 1A) where cleavage at D326 (7,8) and D349 (9) have been reported to generate the N-terminal 36 kDa and 40 kDa fragments respectively. Cleavage at D326 has been reported to be a result of the activity of either caspase-3 or caspase-9, while caspase-6 and caspase-7 exhibit cleavage at both sites (9). Nonetheless, it has been reported that the autophosphorylation at S327 of MST1 limits the cleavage at this site by caspases, with the exception of caspase-3 which does not exhibit significantly attenuated activity for D326 upon autophosphorylation of S327 (10). This last point highlights the interplay between phosphorylation and proteolytic pathways that is emerging to be a widely occurring theme of the apoptotic program (11).
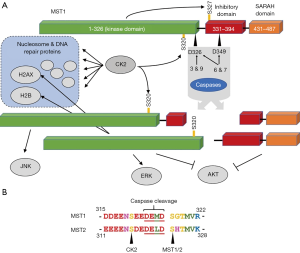
While MST1 and MST2 are typically considered to be redundant kinases as a result of their high sequence homology (78% identity) only one caspase cleavage site is conserved in MST2 (Figure 1B). Like MST1, MST2 also exhibits caspase dependent cleavage during apoptosis (12). While MST2 also has a conserved serine in the P1’ position, the potential for this being a regulatory autophosphorylation site that may regulate caspase cleavage has yet to be investigated.
Caspase cleavage of MST1 separates the N-terminal kinase domain from the C-terminal inhibitory and dimerization domains (13) (Figure 1A), releasing the kinase domain from autoinhibition. The MST1 C-terminal fragment contains a coiled-coil dimerization domain known as the Salvador Ras association (RA) domain family (RASSF)-Hippo (SARAH) domain (14). In addition, the C-terminal fragment contains the autoinhibitory domain (amino acids 331–394) that prevents the access of substrates to the MST1 kinase active site until a conformational change is induced to enable substrate access to the active site (13). The loss of the C-terminal regulatory domain, containing nuclear export signal sequences, leads to the nuclear localization of the N-terminal catalytic domain of MST1 (15) and the induction of cellular processes associated with apoptosis, such as chromatin condensation (7,15,16). In line with the observed chromatin changes as a result of cleaved MST1, identified cellular targets of cleaved MST1 include the phosphorylation of histones H2B and H2AX (17). In addition, the 40 kDa fragment of MST1 has been reported to phosphorylate JNK and p38 while the 38 kDa fragment phosphorylates ERK (18). In addition to contributing positively to the apoptotic program, additional levels of complexity of this proteolytic/phosphorylation signaling axis have also been reported. The cleaved N- and C-terminal fragments of MST1 have also been demonstrated to function in the inhibition of AKT (19) and thus also appears to prevent the inhibition of apoptosis by the activity of AKT (Figure 1A).
A recent report by Servas et al. (6) has identified that the anti-apoptotic CK2 kinase phosphorylates both full length and cleaved MST1 at S320. This observation is not entirely unexpected with CK2 being a prolific kinase with hundreds of known cellular targets (20), nonetheless this discovery leads to potential implications for cross talk between these signaling pathways. While this site of phosphorylation on MST1 by CK2 is in close proximity to the site of caspase cleavage at D326, investigations revealed that this phosphorylation S320 does not impact MST1 cleavage by caspase-3. Whether this phosphorylation impacts the cleavage by other caspases remains to be determined. In addition to the phosphorylation of MST1 by the CK2 holoenzyme, this work demonstrates the ongoing association of these kinases in cells as determined by biochemical fractionations, immunofluorescence and proximity dependent ligation assays.
Furthermore, the resulting function of MST1 phosphorylation and CK2 association remains to be investigated. The PDX1 phosphorylation by MST was demonstrated to be insensitive to the activity of CK2 in in vitro assays. As suggested by the authors, while there was no change to the inherent kinase activity of MST1 in vitro upon phosphorylation and binding by CK2, these interactions may alter substrate recognition in vivo. This scenario may be of particular relevance with regards to nucleosome and other DNA binding proteins (Figure 1A) as both of these kinases as known to phosphorylate these proteins.
While not investigated in this report, it is likely that the same phosphorylation by CK2 is conserved in MST2 as a result of the sequence conservation between these proteins (Figure 1B).
Overall, this reported interaction between MST1 and its caspase cleaved form with CK2 provides insight into the increasing complex networks of phosphorylation and proteolytic signaling networks and may demonstrate to impact the signal axis of both of these kinases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell 2010;19:491-505. [Crossref] [PubMed]
- Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 2014;141:1614-26. [Crossref] [PubMed]
- Chaulk SG, Lattanzi VJ, Hiemer SE, et al. The Hippo pathway effectors TAZ/YAP regulate dicer expression and microRNA biogenesis through Let-7. J Biol Chem 2014;289:1886-91. [Crossref] [PubMed]
- Mori M, Triboulet R, Mohseni M, et al. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell 2014;156:893-906. [Crossref] [PubMed]
- Kurokawa M, Kornbluth S. Caspases and kinases in a death grip. Cell 2009;138:838-54. [Crossref] [PubMed]
- Servas C, Kiehlmeier S, Hach J, et al. The mammalian STE20-like kinase 1 (MST1) is a substrate for the apoptosis inhibiting protein kinase CK2. Cell Signal 2017;36:163-75. [Crossref] [PubMed]
- Graves JD, Gotoh Y, Draves KE, et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J 1998;17:2224-34. [Crossref] [PubMed]
- Lee KK, Murakawa M, Nishida E, et al. Proteolytic activation of MST/Krs, STE20-related protein kinase, by caspase during apoptosis. Oncogene 1998;16:3029-37. [Crossref] [PubMed]
- Graves JD, Draves KE, Gotoh Y, et al. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J Biol Chem 2001;276:14909-15. [Crossref] [PubMed]
- Glantschnig H, Rodan GA, Reszka AA. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J Biol Chem 2002;277:42987-96. [Crossref] [PubMed]
- Dix MM, Simon GM, Wang C, et al. Functional interplay between caspase cleavage and phosphorylation sculpts the apoptotic proteome. Cell 2012;150:426-40. [Crossref] [PubMed]
- Deng Y, Pang A, Wang JH. Regulation of mammalian STE20-like kinase 2 (MST2) by protein phosphorylation/dephosphorylation and proteolysis. J Biol Chem 2003;278:11760-7. [Crossref] [PubMed]
- Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem 1996;271:21049-53. [Crossref] [PubMed]
- Rawat SJ, Chernoff J. Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem Sci 2015;40:149-56. [Crossref] [PubMed]
- Ura S, Masuyama N, Graves JD, et al. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proc Natl Acad Sci U S A 2001;98:10148-53. [Crossref] [PubMed]
- Ura S, Nishina H, Gotoh Y, et al. Activation of the c-Jun N-terminal kinase pathway by MST1 is essential and sufficient for the induction of chromatin condensation during apoptosis. Mol Cell Biol 2007;27:5514-22. [Crossref] [PubMed]
- Wen W, Zhu F, Zhang J, et al. MST1 promotes apoptosis through phosphorylation of histone H2AX. J Biol Chem 2010;285:39108-16. [Crossref] [PubMed]
- Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell Signal 2008;20:892-906. [Crossref] [PubMed]
- Cinar B, Fang PK, Lutchman M, et al. The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J 2007;26:4523-34. [Crossref] [PubMed]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J 2003;17:349-68. [Crossref] [PubMed]
Cite this article as: Leitao LC, Ismail AM, Fahlman RP. The expanding complexity of the phosphorylation and proteolytic signaling axis of MST1. Biotarget 2018;2:9.


